Personal collections


The first law of thermodynamics can be represented by the expression
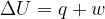
State what is meant by the symbols in the expression.
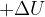 : .....................................................................
: .....................................................................
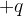 : ............................................................................
: ............................................................................
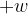 : ..........................................................................
: ..........................................................................
A fixed mass of an ideal gas undergoes a cycle ABCA of changes, as shown in the figure below.
During the change from A to B, the energy supplied to the gas by heating is 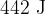 . Use the first law of thermodynamics to show that the internal energy of the gas increases by
. Use the first law of thermodynamics to show that the internal energy of the gas increases by 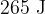 .
.
During the change from B to C, the internal energy of the gas decreases by 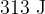 . By considering molecular energy, state and explain qualitatively the change, if any, in the temperature of the gas.
. By considering molecular energy, state and explain qualitatively the change, if any, in the temperature of the gas.
For the change from C to A, use the data in (b)(i) and (b)(ii) to calculate the change in internal energy