Personal collections


A cylinder contains 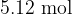 of an ideal gas at pressure of
of an ideal gas at pressure of 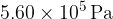 and volume
and volume 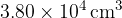 .
.
Determine the temperature of the gas.
The average kinetic energy 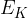 of a molecule of the gas is given by the expression
of a molecule of the gas is given by the expression
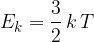
where 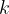 is the Boltzmann constant and
is the Boltzmann constant and 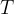 is the thermodynamic temperature. The gas is heated at constant pressure so that its temperature rises by
is the thermodynamic temperature. The gas is heated at constant pressure so that its temperature rises by 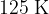 .
.
Use your answer in (a) to determine the new volume of the gas.
Calculate the increase in internal energy of the gas. Explain your working.
Use your answer in (b)(i) to determine the external work done during the expansion of the gas.
Calculate the total thermal energy required to heat the gas in (b).