Personal collections


A fixed amount of helium gas is sealed in a container. The helium gas has a pressure of 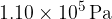 , and a volume of
, and a volume of 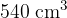 at a temperature of
at a temperature of 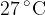 . The volume of the container is rapidly decreased to
. The volume of the container is rapidly decreased to 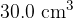 . The pressure of the helium gas increases to
. The pressure of the helium gas increases to 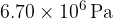 and its temperature increases to
and its temperature increases to 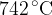 , as illustrated in the figure below.
, as illustrated in the figure below.
No thermal energy enters or leaves the helium gas during this process.
The first law of thermodynamics may be expressed as
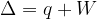
Use the first law of thermodynamics to explain why the temperature of the helium gas increases.