Personal collections


The first law of thermodynamics may be expressed in the form
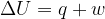
State, for a system, what is meant by:
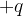 : ..............................................................
: ..............................................................
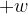 : ..............................................................
: ..............................................................
State what is represented by a negative value of 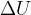 .
.
An ideal gas, sealed in a container, undergoes the cycle of changes shown in the figure below.
At point A, the gas has volume 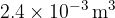 , pressure
, pressure 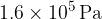 and temperature
and temperature 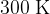 . The gas is compressed suddenly so that no thermal energy enters or leaves the gas during the compression. The amount of work done is
. The gas is compressed suddenly so that no thermal energy enters or leaves the gas during the compression. The amount of work done is 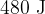 so that, at point B, the gas has volume
so that, at point B, the gas has volume 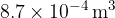 , pressure
, pressure 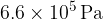 and temperature
and temperature 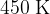 . The gas is now cooled at constant volume so that, between points B and C,
. The gas is now cooled at constant volume so that, between points B and C,  of thermal energy is transferred. At point C, the gas has pressure
of thermal energy is transferred. At point C, the gas has pressure 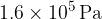 and temperature
and temperature 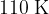 . Finally, the gas is returned to point A.
. Finally, the gas is returned to point A.
Calculate the external work done on the gas during the expansion from point C to point A.
Complete the table below for the changes from:
point A to point B
point B to point C
point C to point A.