Personal collections


The first law of thermodynamics may be represented by the equation
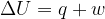
State what is meant by each of the following symbols.
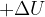 : ...........................................................
: ...........................................................
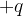 : .............................................................................
: .............................................................................
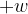 : .............................................................................
: .............................................................................
An amount of 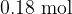 of an ideal gas is held in an insulated cylinder fitted with a piston, as shown in the figure below.
of an ideal gas is held in an insulated cylinder fitted with a piston, as shown in the figure below.
Atmospheric pressure is 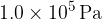 . The volume of the gas is suddenly increased from
. The volume of the gas is suddenly increased from 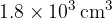 to
to 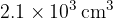 . For the expansion of the gas,
. For the expansion of the gas,
calculate the work done by the gas and hence show that the internal energy changes by 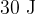 ,
,
determine the temperature change of the gas and state whether the change is an increase or a decrease.