Personal collections


The volume of 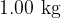 of water in the liquid state at
of water in the liquid state at 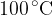 is
is 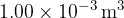 . The volume of
. The volume of 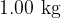 of water vapour at
of water vapour at 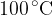 and atmospheric pressure
and atmospheric pressure 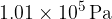 is
is 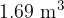 .
.
Show that the work done against the atmosphere when 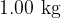 of liquid water becomes water vapour is
of liquid water becomes water vapour is 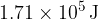 .
.
The first law of thermodynamics may be given by the expression
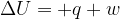
where 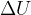 is the increase in internal energy of the system. State what is meant by
is the increase in internal energy of the system. State what is meant by
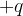 : ..............................................................
: ..............................................................
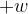 : ..............................................................
: ..............................................................
The specific latent heat of vaporisation of water at 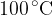 is
is 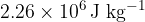 . A mass of
. A mass of 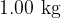 of liquid water becomes water vapour at
of liquid water becomes water vapour at 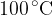 . Determine, using your answer in (a), the increase in internal energy of this mass of water during vaporisation.
. Determine, using your answer in (a), the increase in internal energy of this mass of water during vaporisation.