Personal collections


A fixed mass of gas has an initial volume of 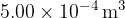 at a pressure of
at a pressure of 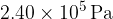 and a temperature of
and a temperature of 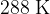 . It is heated at constant pressure so that, in its final state, the volume is
. It is heated at constant pressure so that, in its final state, the volume is 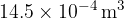 at a temperature of
at a temperature of 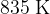 , as illustrated in the figure below.
, as illustrated in the figure below.
The total thermal energy supplied to the gas for this change is 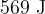 . Determine
. Determine
the external work done,
the change in internal energy of the gas. State whether the change is an increase or a decrease in internal energy.