Personal collections


A constant mass of an ideal gas has a volume of 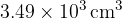 at a temperature of
at a temperature of 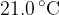 . When the gas is heated,
. When the gas is heated, 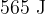 of thermal energy causes it to expand to a volume of
of thermal energy causes it to expand to a volume of 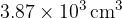 at
at 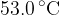 . This is illustrated in the figure below.
. This is illustrated in the figure below.
Show that the initial and final pressures of the gas are equal.
The pressure of the gas is 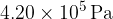 . For this heating of the gas,
. For this heating of the gas,
calculate the work done by the gas,
use the first law of thermodynamics and your answer in (i) to determine the change in internal energy of the gas.