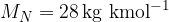Personal collections


In a vessel of a volume of 5 l is a mixture of oxygen 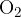 and nitrogen
and nitrogen 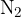 gases. 55% of the total mass of the mixture is oxygen and 45% is nitrogen. The temperature in the vessel is 300 K and the density of the mixture is
gases. 55% of the total mass of the mixture is oxygen and 45% is nitrogen. The temperature in the vessel is 300 K and the density of the mixture is 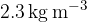 .
.
What is the mass of the mixture?
What is the total pressure in the vessel?
What is the ratio of the number of oxygen molecules and the number of nitrogen molecules in the mixture?
The general gas constant is 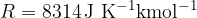
The Avogadro constant is 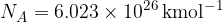
Molar mass of oxygen is 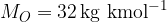
Molar mass of nitrogen is