Personal collections


Initially, gas with a volume of 1 litre is at a pressure of 1.5 bar and a temperature of 70 ° C. We first make an isochoric change on the gas and measure the temperature which is 413 ° C. Then, we make an isothermal change and measure the pressure which is 1 bar. We also make an isobaric change and in the end, the volume of gas is 2 litres. Determine the missing pair between the variables 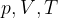 for each gas state in the task.
for each gas state in the task.