Personal collections


The pressure of a bulb is 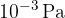 . Assume that all the substance in the bulb is argon gas with a molar mass
. Assume that all the substance in the bulb is argon gas with a molar mass 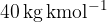 and that the bulb has the shape of a sphere with a radius of 3 cm. When the light is off, the temperature inside the bulb is 300 K.
and that the bulb has the shape of a sphere with a radius of 3 cm. When the light is off, the temperature inside the bulb is 300 K.
How many argon atoms are in the bulb?
When the light is turned on, the gas in the bulb heats up to 380 K. What is the pressure in the bulb then?
Note that the general gas constant is 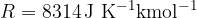 and the Avogadro constant is
and the Avogadro constant is 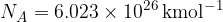 .
.