Personal collections


In a sealed and vacuum closed metal box with a volume of 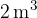 , we supply various gases stored in separate cylinders. From a cylinder in which oxygen is stored at a pressure of 20 bar and a temperature of 90 ° C, 12 l of oxygen is delivered.
, we supply various gases stored in separate cylinders. From a cylinder in which oxygen is stored at a pressure of 20 bar and a temperature of 90 ° C, 12 l of oxygen is delivered. 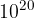 molecules are introduced from the nitrogen cylinder and 40 kg of gas from the helium cylinder. We also wait for the gases to mix well and stabilize at a temperature of 20 ° C. What is the pressure in the box?
molecules are introduced from the nitrogen cylinder and 40 kg of gas from the helium cylinder. We also wait for the gases to mix well and stabilize at a temperature of 20 ° C. What is the pressure in the box?