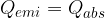Personal collections


A mountaineer goes to a mountain hut on a cold winter morning.
He takes beech logs and lights the stove. He can feel the heat emitted by the open fire. Beech logs emit heat when burning, which is first perceived as thermal radiation. The heat is gradually transferred from the firebox to the entire stove. This happens through a process called heat conduction. When the air around the stove heats up, it rises because it is less dense than the cold air in the surroundings. It cools on the ceiling and falls again. The air circulates around the room and heats it evenly. The phenomenon is called convection.
Unfortunately, the heat from the hut escapes through the walls, windows, and roof into the surroundings. We want these losses to be as small as possible and therefore, we make sure that the hut is thermally insulated. Wooden walls are a good thermal insulator. The snow on the roof also helps to better insulate the hut.
The heat is therefore emitted by the wood burning in the stove. In order for the mountaineer to have a constant temperature in the hut, he must constantly add fuel to the fire. This will replace the heat escaping to the surroundings.
Let's discuss in more detail, the phenomena mentioned above.
We will learn the difference between heat and temperature, what heat is, what a heat source is, and how heat is transferred to the surroundings. We will also calculate how much heat is needed to heat a substance to the desired temperature.
Heat is a form of energy that is transferred between bodies.
Heat is always transferred:
from a warmer body
to a colder body.
We denote it by the letter 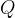 . Its unit is Joule (
. Its unit is Joule (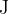 ).
).
Heat transfers internal energy between bodies. By adding heat, we increase the internal energy of a body, and by removing heat, we decrease it. Heat therefore directly affects the change in internal energy 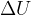 of bodies:
of bodies:
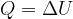
A heated (warm) body is not said to have heat. Remember, a heated body has internal energy. To warm a body, we supply it with heat. We say that the body has absorbed heat. As a result, its internal energy increases. As the internal energy increases, so does the temperature of the body.
Internal energy is discussed more thoroughly in the material, Internal energy for KS4.
Let’s look at two examples where we can learn more precisely the relationships between heat, temperature, and internal energy.
When a substance absorbs or emits heat, its internal energy changes:
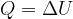
Due to the supply or removal of heat, the temperature of the substance or its physical state changes.
It is very important to distinguish between heat and temperature, as the two concepts sound similar but they have a completely different role. Let's highlight the difference:
Temperature is not energy
Temperature is just a quantity that indicates the current thermal state of a substance. If we know the temperature and the physical state of a substance, we can calculate its internal energy.
The calculation of internal energy is beyond the scope of this material.
Since temperature is not energy, its magnitude is not conserved and it does not flow. It is not flowing anywhere like energy. It only changes and thus indicates that the internal energy is also changing.
Heat is energy
Heat is, therefore, like any energy, transferred between bodies and flowing:
from a body with a higher temperature,
to a body with a lower temperature.
The heat absorbed increases the internal energy and the heat emitted decreases it.
Let's distinguish between heat and temperature:
Heat is energy that is transferred from warmer bodies to colder ones. Its unit is J (Joule).
Temperature indicates the thermal state of a substance and is measured in 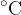 or K (Kelvin).
or K (Kelvin).
A heat source is any heated body. This body emits heat to the surroundings and to other cooler bodies in the surroundings. We know that heat always flows from an area with a higher temperature to an area with a lower temperature.
Let’s take a look at the two biggest heat sources on our planet. The Earth gets most of its heat from the Sun. This energy is called solar energy. The Sun radiates heat and the Earth absorbs it.
Another major source of heat is the heat coming from the Earth's interior. This is called geothermal energy. It is formed by the radioactive decay of elements and keeps the Earth's core liquid due to its high temperature.
Most of the heat sources we use on Earth were created as a result of the action of the Sun.
You can learn more about energy sources in the chapter, Energy Sources.
The heat we need in everyday life is mostly obtained in two ways:
By burning
Combustion is a chemical process in which heat is generated. In doing so, we use certain energy sources like gas, oil, gasoline, coal, wood, etc. The quantity of heat a substance emits during combustion depends on its mass and the type of substance. We are usually interested in how many Joules of heat are produced if 1 kg of a substance burns completely.
By means of electrical power
We also use electricity as a source of heat. An electric stove, heater, or cooker converts electrical work into heat with the help of an electric current.
The heat  emitted by the electric heater is exactly the same as the electrical work done and this is equal to the product of the power
emitted by the electric heater is exactly the same as the electrical work done and this is equal to the product of the power 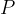 of the heater and time
of the heater and time 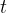 taken to pass the heat
taken to pass the heat
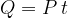
The power of an electric heater is usually written on the product itself. By measuring the time, we can therefore accurately calculate the quantity of heat emitted by the heater.
Heat transfer is the exchange of the internal energy of bodies. Heat can pass from a source to another substance in three ways:
conduction,
convection,
and radiation.
The above three methods of heat transfer are also called heat transfer mechanisms. Let's take a look at them.
Heat naturally flows from a warmer body to a colder one. Molecules in a heated body move faster than in a colder body. Faster molecules hit slower ones and give them their kinetic energy. In solids, the distances between molecules are smaller than in liquids and gases. Kinetic energy is therefore more easily transferred to slower molecules by collisions. This is why solids (especially metals) are usually better conductors of heat than liquids and gases.
Heat also flows within a single body. When we heat a metal rod in one part, heat travels from the warmer part of the rod (red colour) to the cooler part (blue colour).
Substances conduct heat differently. Good heat conductors are, for example, metals.
We many times wish that the substance would conduct heat as poorly as possible. Substances that conduct heat poorly are called thermal insulators. Among them is wood. Good thermal insulators are gases and liquids, as well as solids that contain a lot of air, for example:
styrofoam,
siporex,
brick etc.
A good conductor of heat transfers a lot of heat by conduction. Metals are good heat conductors. For a poor conductor, little heat is transferred by conduction. We call poor conductors insulators. Gases and liquids are good insulators.
Convection is a method by which heat is transferred through gases and liquids. When a gas or liquid in direct contact with a heat source is heated, it expands. Its volume increases. Therefore, its density decreases. Upthrust causes the heated liquid or gas to rise. At a height, however, it cools down and falls again. The process is repeated in the form of the circulation of matter. We call this convection.
Gases and liquids are mostly poor conductors of heat. Heat transfer by conduction is not effective in these cases. Nevertheless, the air in the room and the soup in the pot heat up quickly due to convection. In gases and liquids, convection is the most efficient heat transfer mechanism.
Heat can also travel in a vacuum, such as space. The phenomenon is called radiation. Heat is transferred in this case by heat rays. They have the same nature as light rays or radio waves. They are called infrared rays. They propagate at the same speed as light, i.e. 300,000 km/s. Thermal radiation is high if the temperature of the radiating object is high. It also depends on the colour of the body. A black body radiates best.
The bodies on which the radiant rays fall are heated. The rays are absorbed by the body on which they fall. The absorption of heat rays depends on the colour of the body on which they fall. The black body absorbs heat best and also radiates best.
Bodies that have a lighter colour absorb less sunlight than darker bodies. Bright bodies reflect more light, while darker bodies absorb more. Because of this, darker bodies heat up more in the sun.
In this chapter:
we will first learn how to calculate how much heat we need to add to a substance to heat it to a certain temperature. Here we will learn what the specific heat capacity of a substance is.
we will then thermally mix two same or different substances that are at different temperatures and wait for the temperatures to be equal. We will then calculate the final temperature of the mixture.
We have learned that by adding heat to a substance, the temperature increases (or the physical state changes). How much the temperature will rise during heating depends on the:
type and
mass
of the substance.
Let's repeat the above experiment by pouring twice as much water into the container. In this case, we need twice as much heat for the same temperature difference. Instead of water, we can take 1 kg of some other substance. From the results of the measurements, we understand how many Joules (J) of heat are needed to heat a kilogram of the substance by 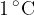 or 1 K.
or 1 K.
The results of the measurements can be summarized as follows:
The heat quantity 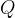 required to increase the temperature of a substance by
required to increase the temperature of a substance by 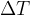 is directly proportional to the mass
is directly proportional to the mass 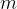 of the substance. The proportionality factor is called the specific heat capacity and is denoted by
of the substance. The proportionality factor is called the specific heat capacity and is denoted by  :
:
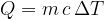
Let's obtain the expression for the specific heat capacity  from the formula above:
from the formula above:
If we insert units for individual quantities in the above equation, we get:
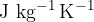
Specific heat capacity  tells us how many Joules (J) of heat we need to raise the temperature of 1 kg of a substance by
tells us how many Joules (J) of heat we need to raise the temperature of 1 kg of a substance by 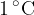 .
.
The heat required to raise the temperature of 1 kg of a substance by 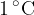 is different for each substance - but precisely determined. From the above experiment, we have already established the specific heat capacity of water:
is different for each substance - but precisely determined. From the above experiment, we have already established the specific heat capacity of water:
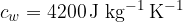
The table below shows the specific heat capacity of some substances:
If heat is added to a substance, its temperature increases, and when heat is removed, the temperature decreases. Heat and temperature change are related by the equation:
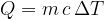
The specific heat capacity  depends on the substance. It tells us how many Joules (J) of heat are needed to heat 1 kg of a substance by
depends on the substance. It tells us how many Joules (J) of heat are needed to heat 1 kg of a substance by 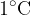 or 1 K.
or 1 K.
Two identical or different substances that are at different temperatures are placed in the same thermally insulated container. We wait for the temperature to stabilize at a temperature that is somewhere between the temperature of:
the hot body and
the cold body.
Let’s call it the final steady temperature and we denote it as 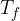 . When the temperatures of the two substances become equal, thermal equilibrium is established.
. When the temperatures of the two substances become equal, thermal equilibrium is established.
Before thermal equilibrium is established, heat gradually flows from the hot body to the cold body. This cools the hot body and warms the cold one. In the end, the two temperatures of the bodies become equal at the final temperature.
It is also possible for the heat of a substance to be used to change the physical state of another substance. For example, ice melts into water on contact with a warmer object, water evaporates on contact with a hot object. Such cases are discussed in the material, Thermal Equilibrium.
The quantity of heat 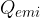 emitted by the hot body is equal to the quantity of heat
emitted by the hot body is equal to the quantity of heat 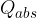 absorbed by the cold body. Let's write this mathematically as:
absorbed by the cold body. Let's write this mathematically as:

Let the hot body have a mass 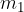 , specific heat capacity
, specific heat capacity 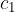 and initial temperature
and initial temperature 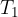 . When it cools down to a final temperature
. When it cools down to a final temperature 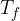 , it emits heat:
, it emits heat:
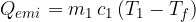
Let the cold body have a mass of 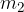 , specific heat capacity
, specific heat capacity 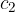 , and an initial temperature of
, and an initial temperature of 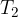 . When heated to a final temperature of
. When heated to a final temperature of 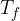 , it absorbs heat:
, it absorbs heat:
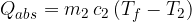
Therefore, equation 1 becomes:
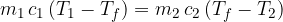
If two substances with different temperatures are placed in a thermally insulated container, the warmer substance cools to the final temperature, and the colder substance heats up to the same temperature. The heat 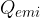 emitted by the warmer substance is equal to the heat
emitted by the warmer substance is equal to the heat 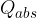 absorbed by the cooler substance:
absorbed by the cooler substance: