Personal collections


Life without electrical appliances is hard to imagine today. Electrical devices are powered by an electric charge. It enters them and flows in them through wires.
The basic properties of electric charge can be observed in the phenomena of static electricity. We often encounter static electricity in our daily lives. Let's look at some examples.
The examples described above are phenomena that often occur between bodies where an electric charge is present. Next, let’s take a look at where the charge comes from and how bodies are charged.
The electric charge is hidden in the atoms, of which each body is composed. In order to better understand the electric charge, we must therefore take a closer look at the structure of the atom itself. An atom consists of the:
atomic nucleus and
electron cloud.
The atomic nucleus consists of:
protons,
neutrons,
which are firmly interconnected.
In the electron cloud, however, electrons move.
An electric charge, like mass, is one of the properties of particles in an atom (basic particles), namely:
protons have a positive charge,
electrons have a negative charge,
neutrons, however, have no charge.
The charge carriers are therefore electrons and protons, as they have their own electric charge.
Experiments have shown that all electrons and protons have the same charge. This charge is called the elementary charge and is denoted by  . The elementary charge is measured with the unit Coulomb, denoted by
. The elementary charge is measured with the unit Coulomb, denoted by 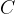 .
.
The electric charge is the same in an electron and a proton:
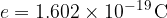
The charges on protons and electrons are distinguished by signs, as electrons carry a negative charge and protons carry a positive charge:
The charge on an electron is:
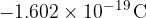
The charge on a proton is:
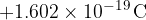
Atoms usually have the same number of electrons and protons. However, because the charge on an electron is of the opposite sign to that on a proton, the total electric charge of an atom is equal to 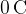 . This means that an atom is electrically neutral.
. This means that an atom is electrically neutral.
Like atoms, the bodies around us are usually electrically neutral. However, they can become charged. Let’s look at how and why charges occur.
Each body is made up of atoms. In atomic nuclei, protons and neutrons are strongly linked together. However, electrons moving around atomic nuclei are quite easy to extract from the atoms.
If it happens that the body has excess or deficiency of electrons, then it is no longer electrically neutral. Then the body is charged or electrically charged. Because we have both negative and positive charges, a body can be:
Negatively charged
A body is negatively charged if it has an excess of electrons. Then it has more electrons than protons, so the negative charge is more than the positive charge.
Positively charged
A body is positively charged if it has an electron deficiency. Then it has fewer electrons than protons, so the positive charge is more than the negative charge.
A body can be charged by rubbing. In doing so, it is charged positively or negatively.
Which body will emit electrons and which will receive depends on the combination of materials we rub against each other. Therefore, we cannot know in advance which body will be charged negatively and which positively.
By agreement, it is considered that:
a plastic rod rubbed against a woolen cloth is charged negatively.
a glass rod rubbed against a silk cloth is charged positively.
We have seen that when a body is charged, electrons come from one body to another, bringing their charges to the body. Therefore, the charge on a body depends on the number of electrons, which:
come on the body or
leave it.
The electric charge of a body is denoted by the letter 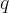 . The more electrons the body receives or emits, the greater its charge.
. The more electrons the body receives or emits, the greater its charge.
The body charge is calculated by the equation:
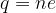
where n is the number of electrons received or emitted and  is the elementary charge.
is the elementary charge.
If the body accepts electrons, its charge is negative. If it gives them away, however, its charge is positive.
All substances are roughly either conductors or insulators depending on either electric charge:
can easily move through them or
cannot move.
An electrical conductor is a substance that allows electric charge to flow through it while an electrical insulator is the one that does not allow electric charge to flow through it.
Both insulators and conductors can be charged, but not in the same way. Let's see how we charge them.
Electrical insulators are substances through which electrons cannot move. They are bound to individual atoms. This means that the charge on the body is static - it stays in place.
In the previous case, we have already listed two materials that are electrical insulators:
wool (woolen cloth),
plastic (plastic comb).
Insulators can be charged by rubbing against other insulators. While rubbing, we tear off a few electrons from one body, which then pass to another body. In doing so, one body is charged positively and the other negatively.
By rubbing the two insulators, both bodies are charged with the same amount but opposite charges.
Because electrons cannot move freely in an insulator, the body is charged at the point where we extract or add electrons to it. This means that after charging, all the charges are gathered on the surface of the insulator.
Electrical conductors are substances through which electrons move freely. The electric charge can therefore flow through the body.
To charge conductive bodies, a touch of another charged body is enough.
The conductor is charged with a charge of the same type as the body from which the charge came.
Even when the conductor is charged, all the charges remain on its surface. However, the charges are evenly distributed over the entire surface of the conductor. We will find out the reason for this phenomenon in the material, Electric Force for KS4.
The conductor cannot be charged by rubbing, as all the extracted charges are instantly transferred back to where they came from.