Personal collections


Light is an electromagnetic wave. Electromagnetic (EM) waves comprise different wavelengths or frequencies. All they have in common is the speed of the waves. This is about 300,000 km/s in the air and in empty space.
The light received by the Earth from the outside is largely emitted by the Sun. Different wavelengths are represented in this light. Which wavelengths are more or less represented is determined by the surface temperature of the Sun.
Knowing the radiant temperature of the Sun (approximately 5500 K), we could calculate the wavelengths with the help of Planck's law, which we will not discuss in this material. At this temperature, the Sun emits the strongest EM wavelengths that we perceive as visible light. It also emits invisible heat waves (infrared waves) and also invisible and more dangerous ultraviolet waves.
In addition to the Sun, we also have other, artificial sources of electromagnetic waves. These are radio transmitters, lamps, high-frequency radiation sources, etc. If we limit ourselves to light waves, their radiation can be compared to the radiation of the Sun.
When we say that the radiant temperature of a light source is 5400 K, it means that it emits white light composed of a multitude of wavelengths, similar to the Sun. If the lamp is a laser, it emits light of a single wavelength. We call it monochromatic (single color) light. Due to the high energy gathered around a single frequency, it is dangerous and can cause eye damage.
The speed of light in airless space is the same as for all electromagnetic waves:
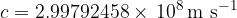
We usually round it to:
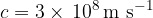
It is approximately the same in the air.
If light passes through matter, its speed decreases. The refractive index indicates how many times it decreases in a given substance relative to air.
Figure 1 below shows the distribution of EM waves regardless of the source of the wave, from very large wavelengths (long waves) to wavelengths of the order of pm (X-rays).
Waves of visible light are also part of this wave. These are sine waves with a wavelength of 750 nm (red light) to 380 nm (violet light). The wavelength 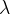 and frequency
and frequency 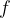 of these waves are related by the equation:
of these waves are related by the equation:
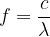
Let's look at the table of colours of light and its wavelengths:
Light sources are:
bodies heated to a high temperature,
bodies that emit light based on the changes in the energy levels of the atom.
Every heated body radiates. At lower temperatures, they emit heat, the infrared rays. If we heat the body to a higher temperature, it emits visible and ultraviolet light in addition to infrared rays.
In the material, Heat transfer, we saw that the strongest radiation occurs in a black body. At the time, we were only thinking of infrared or thermal radiation, but the finding also applies to visible and ultraviolet light radiation.
The light spectrum tells us which wavelengths of light (colours) are emitted by a heated black body. The spectrum shows us the dependence of the power density per unit wavelength interval on the wavelength of the emitted light.
In black body radiation, the spectrum is continuous. Bodies radiate at all wavelengths, but some more than others. The spectrum could be calculated using Planck's law. At which wavelength  is the peak of the spectrum depends on the temperature
is the peak of the spectrum depends on the temperature 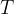 of the body and this is given by the formula:
of the body and this is given by the formula:
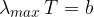
where 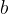 is the Wien's constant which is given as:
is the Wien's constant which is given as:
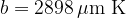
Figure 3 shows the radiant energy density of a black body heated to different temperatures: 5500 K, 4500 K, and 3500 K. The left part of the graph represents the ultraviolet part of the spectrum, the central part, the visible light spectrum (rainbow colours are plotted), and the right part, the infrared part of the spectrum:
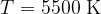
This is the approximate radiant temperature of the Sun. The peak of the spectrum represents the radiation of visible light.
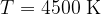
The radiant energy density decreases and the peak of the spectrum moves towards the infrared region. It has a peak at a wavelength of 640 nm.
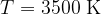
This is approximately the radiant temperature of a candle. Most of the spectrum is in the infrared range (IR) light, only a small part is visible light. The peak of the spectrum is at a wavelength of 830 nm.
A black body emits a continuous spectrum of light. All frequencies are represented in the spectrum, but they are emphasized differently. The peak of the spectrum depends on the temperature. At lower temperatures, the body emits mainly thermal (IR) rays, at higher temperatures also visible and ultraviolet (UV) rays. At 5500 (the radiant temperature of the Sun), the peak of the spectrum is in the area of visible light.
Lamps that emit light due to changes in the atomic states of an atom are called lamps. These are fluorescent lamps, light-emitting diodes (LEDs) lamps, laser diodes, etc. To learn more about the physical background of this radiation, see the material - Energy levels of Atoms.
The lamps do not have a continuous spectrum of light but emit light of individual wavelengths. By combining materials that emit light of different wavelengths, we can obtain light similar to the light emitted by a black body. The only difference is that they can emit only visible light and not heat, like incandescent bulbs. Because they save energy, so they are also called energy-saving lamps.
In the case of lamps, we can only talk about the equivalent colours temperature. This is the temperature of a black body that would emit a similar spectrum of light as the observed lamp.
Laser diodes emit light of a single wavelength. We call it coherent, monochromatic light. The laser beam does not scatter with distance from the lamp and therefore transmits a large amount of radiant energy in a small cross-section. It is therefore dangerous to the eyes.
The laser beam is used for:
measurements (laser distance and speed meters),
creating three-dimensional (3D) images - holograms, in industry, medicine, and
transmitting information in telecommunications.
Luminosity in watts is the energy 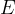 emitted by a light body per unit time
emitted by a light body per unit time 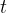 .
.
It is similar to luminous power 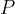 or luminous flux
or luminous flux 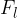 , which is equivalent to the total wavelength-weighted power emitted by a source and its unit is the lumen (lm).
, which is equivalent to the total wavelength-weighted power emitted by a source and its unit is the lumen (lm).
Almost the entire luminous flux of a light bulb goes into light radiation. In conventional incandescent lamps, only a small part of the emitted light is visible light, infrared (thermal) rays predominate.
A lamp can radiate in all directions or just at a certain angle. If we observe radiation only at a certain angle, we use the solid angle 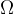 . The unit of a solid angle is steradian, abbreviated as "ster." A solid angle of 1 ster is the angle which has its vertex in the centre of a sphere of radius 1 m and which limits the area of the sphere to the spherical cap of a surface area
. The unit of a solid angle is steradian, abbreviated as "ster." A solid angle of 1 ster is the angle which has its vertex in the centre of a sphere of radius 1 m and which limits the area of the sphere to the spherical cap of a surface area 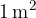 .
.
The area 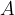 on the sphere illuminated by the lamp in the centre of the sphere at a solid angle
on the sphere illuminated by the lamp in the centre of the sphere at a solid angle 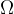 is given as:
is given as:

If the solid angle is smaller, the lamp emits more light only in a certain direction. We say that the luminous intensity 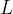 increases in this direction and this is given as the luminous power
increases in this direction and this is given as the luminous power 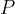 divided by the solid angle
divided by the solid angle 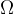 :
:
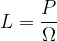
The luminous intensity is denoted by 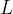 and the unit is W/ster.
and the unit is W/ster.
However, we also have a physiological unit for the luminous intensity which takes into account only the visible part of the spectrum and the fact that the eye is not equally sensitive to all colours. This is the reason that in practice we prefer to use the physiological unit lumen, abbreviated as lm, rather than watts.
Therefore, the luminous intensity 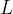 is given in terms of the luminous flux
is given in terms of the luminous flux 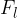 as:
as:

The luminous intensity unit, in this case, is the lumen (lm) over the steradian (ster). It is called a candela - abbreviated as cd. This is one of the basic units in the International System of Units - SI.
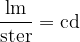
In practice, the illuminance 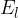 is also used and this is given as the luminous flux
is also used and this is given as the luminous flux 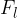 per unit area
per unit area 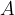 :
:
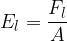
Inserting equations 1 and 2 above, we have:
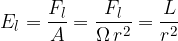
The unit is the lumen per square meter. It is a measure of brightness. The resulting unit is called lux, abbreviated as lx.
Average illuminance values are:
corridors, warehouses 50 lx
laboratory 750 lx
operation halls, fine mechanics 2000 lx
How many lumens a lamp with a certain power gives us depends on the lamp itself. For about 60 to 150 lumens we need:
classical bulb: 8 to 10 W,
fluorescent lamps 6 W,
light bulbs (LED) 1 W.
We perceive light with the help of the eye. On the retina of the eye are cells called rods. These are light receivers that can detect very low light and display it as a colourless light-dark image. During the night, the eye is most sensitive to light with a wavelength of about 550 nm. With this light, the eye can still perceive an illuminance (i.e. the luminous power per square meter of the surface) of approximately:
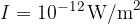
The maximum sensitivity of the eye to light is comparable to the maximum sensitivity of the ear to sound - that is, at frequencies around 1000 Hz.
There are also colour-sensitive cells on the retina called cones. The macula, which is the indentation in the retina opposite the lens, contains many cones. In humans, they enable colour vision. The human eye can perceive colours only in the case of sufficiently strong light. The maximum sensitivity of the eye is for yellow-green light with a wavelength of 555 nm. The cones have three types of pigments that are "tuned" to three wavelengths. The sensitivity peaks of individual types of cones are at 420 - 440 nm (S - short), 530 - 540 nm (M - middle), and 560 - 580 nm) (L - long).
This is a three-colour (trichromatic) viewing system. It is characteristic of some primates and humans. Many other primates and mammals are two-colour (dichromatic) with very limited or no colour perception. Some animals (tropical fish, birds) even have a four-colour vision, as they live in a colour-intensive environment where accurate colour differentiation is essential for survival.
Any colour can be obtained by mixing different intensities of the three primary colours of light: red, green, and blue (RGB). Colour TV or computer monitors take advantage of this.
The colour impression gives us the common response of all three types of cones to incident light. White color can be obtained by mixing the three basic colors or by mixing all the colors (wavelengths) emitted by the sun. We call this the three-color additive model of color perception. Different combinations of light of all possible wavelengths can produce the same response and the same perception of color.