Personal collections


The pressure 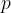 of an ideal gas having density
of an ideal gas having density 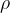 is given by the expression
is given by the expression
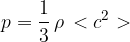
State what is meant by:
an ideal gas
the symbol 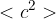 .
.
A cylinder contains a fixed mass of a gas at a temperature of 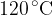 . The gas has a volume of
. The gas has a volume of 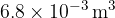 at a pressure
at a pressure 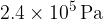 .
.
Assuming the gas acts like an ideal gas, show that the number of atoms of gas in the cylinder is 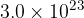 .
.
Each atom of the gas, assumed to be a sphere, has a radius of 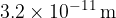 . Use the answer in (i) to estimate the actual volume occupied by the gas atoms.
. Use the answer in (i) to estimate the actual volume occupied by the gas atoms.
One of the assumptions of the kinetic theory of gases is related to the volume of the atoms. State this assumption. Explain whether your answer in (ii) is consistent with this assumption.