Personal collections


The temperature 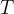 of an ideal gas increases from
of an ideal gas increases from 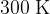 to
to 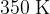 in a large container.
in a large container.
Explain how the movement of the gas molecules causes pressure in the container.
The temperature of a gas depends on the root-mean-square (r.m.s.) speed of its molecules. Calculate the ratio:
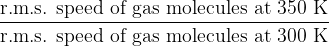 .
.