Personal collections


State what is meant by an ideal gas.
A fixed amount of helium gas is sealed in a container. The helium gas has a pressure of 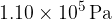 , and a volume of
, and a volume of 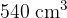 at a temperature of
at a temperature of 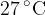 . The volume of the container is rapidly decreased to
. The volume of the container is rapidly decreased to 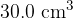 . The pressure of the helium gas increases to
. The pressure of the helium gas increases to 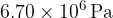 and its temperature increases to
and its temperature increases to 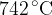 , as illustrated in the figure below.
, as illustrated in the figure below.
The average translational kinetic energy 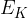 of a molecule of an ideal gas is given by
of a molecule of an ideal gas is given by
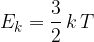
where 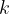 is the Boltzmann constant and
is the Boltzmann constant and 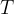 is the thermodynamic temperature. Calculate the change in the total kinetic energy of the molecules of the helium gas.
is the thermodynamic temperature. Calculate the change in the total kinetic energy of the molecules of the helium gas.