Personal collections


State the basic assumptions of the kinetic theory of gases.
Use equations for the pressure of an ideal gas to deduce that the average translational kinetic energy 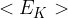 of a molecule of an ideal gas is given by the expression
of a molecule of an ideal gas is given by the expression
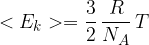
where 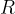 is the molar gas constant,
is the molar gas constant, 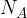 is the Avogadro constant and
is the Avogadro constant and 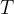 is the thermodynamic temperature of the gas.
is the thermodynamic temperature of the gas.
A deuterium nucleus 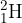 and a proton collide. A nuclear reaction occurs, represented by the equation
and a proton collide. A nuclear reaction occurs, represented by the equation
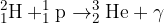
State and explain whether the reaction represents nuclear fission or nuclear fusion.
For the reaction to occur, the minimum total kinetic energy of the deuterium nucleus and the proton is 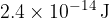 . Assuming that a sample of a mixture of deuterium nuclei and protons behaves as an ideal gas, calculate the temperature of the sample for this reaction to occur.
. Assuming that a sample of a mixture of deuterium nuclei and protons behaves as an ideal gas, calculate the temperature of the sample for this reaction to occur.