Personal collections


One assumption of the kinetic theory of gases is that gas molecules behave as if they are hard, elastic identical spheres. State two other assumptions of the kinetic theory of gases.
Using the kinetic theory of gases, it can be shown that the product of the pressure 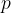 and the volume
and the volume 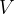 of an ideal gas is given by the expression
of an ideal gas is given by the expression
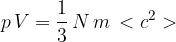
where 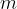 is the mass of a gas molecule.
is the mass of a gas molecule.
State the meaning of the symbol
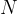 ,
,
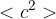 .
.
Use the expression to deduce that the mean kinetic energy 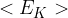 of a gas molecule at temperature
of a gas molecule at temperature 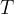 is given by the equation
is given by the equation
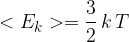
where 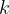 is a constant.
is a constant.