Personal collections


An ideal gas has volume 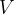 and pressure
and pressure 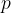 . For this gas, the product
. For this gas, the product 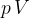 is given by the expression
is given by the expression
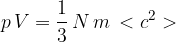
where 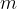 is the mass of a molecule of the gas.
is the mass of a molecule of the gas.
State the meaning of the symbol
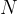 ,
,
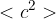 .
.
A gas cylinder of volume 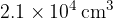 contains helium-4 gas at pressure
contains helium-4 gas at pressure 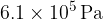 and temperature
and temperature 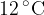 . Helium-4 may be assumed to be an ideal gas.
. Helium-4 may be assumed to be an ideal gas.
Determine, for the helium gas,
the amount, in mol,
the number of atoms.
Calculate the root-mean-square (r.m.s.) speed of the helium atoms.