Personal collections


Explain qualitatively how molecular movement causes the pressure exerted by a gas.
The density of neon gas at a temperature of 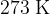 and a pressure of
and a pressure of 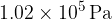 is
is 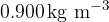 . Neon may be assumed to be an ideal gas. Calculate the root-mean-square (r.m.s.) speed of neon atoms at
. Neon may be assumed to be an ideal gas. Calculate the root-mean-square (r.m.s.) speed of neon atoms at
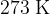 ,
,
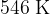 .
.
The calculations in (b) are based on the density for neon being 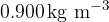 . Suggest the effect, if any, on the root-mean-square speed of changing the density at constant temperature.
. Suggest the effect, if any, on the root-mean-square speed of changing the density at constant temperature.