Personal collections


The pressure 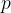 of an ideal gas is given by the expression
of an ideal gas is given by the expression
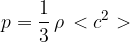
where 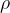 is the density of the gas.
is the density of the gas.
State the meaning of the symbol 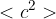 .
.
Use the expression to show that the mean kinetic energy 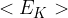 of the atoms of an ideal gas is given by the expression
of the atoms of an ideal gas is given by the expression
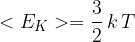
Explain any symbols that you use.
Helium-4 may be assumed to behave as an ideal gas. A cylinder has a constant volume of 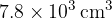 and contains helium-4 gas at a pressure of
and contains helium-4 gas at a pressure of 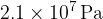 and at a temperature of
and at a temperature of 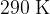 . Calculate, for the helium gas,
. Calculate, for the helium gas,
the amount of gas,
the mean kinetic energy of the atoms,
the total internal energy.