Personal collections


The kinetic theory of gases is based on some simplifying assumptions. The molecules of the gas are assumed to behave as hard elastic identical spheres. State the assumption about ideal gas molecules based on
the nature of their movement,
their volume.
A cube of volume 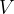 contains
contains 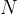 molecules of an ideal gas. Each molecule has a component
molecules of an ideal gas. Each molecule has a component 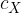 of velocity normal to one side S of the cube, as shown in the figure below.
of velocity normal to one side S of the cube, as shown in the figure below.
The pressure 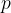 of the gas due to the component
of the gas due to the component 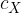 of velocity is given by the expression
of velocity is given by the expression
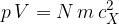
where 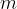 is the mass of a molecule.
is the mass of a molecule.
Explain how the expression leads to the relation
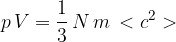
where 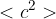 is the mean square speed of the molecules.
is the mean square speed of the molecules.
The molecules of an ideal gas have a root-mean-square (r.m.s.) speed of 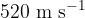 at a temperature of
at a temperature of 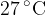 . Calculate the r.m.s. speed of the molecules at a temperature of
. Calculate the r.m.s. speed of the molecules at a temperature of 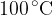 .
.