Personal collections


An ideal gas is assumed to consist of atoms or molecules that behave as hard, identical spheres that are in continuous motion and undergo elastic collisions. State two further assumptions of the kinetic theory of gases.
Helium-4 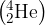 may be assumed to be an ideal gas.
may be assumed to be an ideal gas.
Show that the mass of one atom of helium-4 is 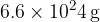 .
.
The mean kinetic energy 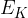 of an atom of an ideal gas is given by the expression
of an atom of an ideal gas is given by the expression
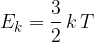
Calculate the root-mean-square (r.m.s.) speed of a helium-4 atom at a temperature of 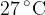 .
.