Personal collections


Describe the motion of molecules in a gas, according to the kinetic theory of gases.
Describe what is observed when viewing Brownian motion that provides evidence for your answer in (a).
At a pressure of 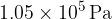 and a temperature of
and a temperature of 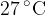 ,
, 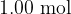 of helium gas has a volume of
of helium gas has a volume of 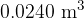 . The mass of
. The mass of 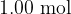 of helium gas, assumed to be an ideal gas, is
of helium gas, assumed to be an ideal gas, is 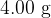 .
.
Calculate the root-mean-square (r.m.s.) speed of an atom of helium gas for a temperature of 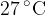 .
.
Using your answer in (i), calculate the r.m.s. speed of the atoms at 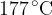 .
.