Personal collections


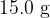 of water at
of water at 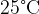 is dropped onto a large block of ice at
is dropped onto a large block of ice at 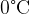 . The water cools to
. The water cools to 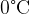 and some of the ice melts. Assume that all the energy lost by the water is gained by the ice. What is the mass of ice that melts if the specific heat capacity of water is
and some of the ice melts. Assume that all the energy lost by the water is gained by the ice. What is the mass of ice that melts if the specific heat capacity of water is 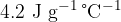 and the specific latent heat of fusion of ice is
and the specific latent heat of fusion of ice is 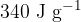 .
.