Personal collections


Nuclear reactions are reactions in which nuclear forces play a key role. In this way, they differ from chemical reactions that take place due to electromagnetic forces between atoms and molecules. Nuclear reactions are initiated by collisions with external particles and are therefore different from radioactive decay which takes place without external intervention. The result of such collisions is modified nuclei or particles.
In general, we describe a nuclear reaction with an equation such as:
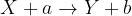
where:
X and Y are the initial and final core elements respectively, while
a and b are the initiators and various products of the reaction.
A shorter expression of such a reaction is given as:
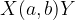
Conservation laws apply to all nuclear reactions. The following are conserved:
Total energy - mass
As we know, mass and energy are not conserved by themselves (see mass defect). But the total energy is always conserved.
Electric charge
The total electric charge of all reactants is always equal to the total charge of all products.
Number of nucleons
This reaction is unusual at first glance. The reason for this is deeper conservation laws since protons and neutrons are themselves composite particles.
Momentum
Both laws known from classical physics also apply to nuclear reactions:
In nuclear reactions, the following are conserved:
number of nucleons,
electric charge,
total energy - mass.
According to the energy balance, nuclear reactions can be divided into:
Exothermic: In these reactions, energy is released.
Endothermic. In these reactions, energy is absorbed.
Let's discuss them.
For exothermic reactions, the following applies:
energy is released, and
as a result, the total mass of the products formed is less than the total mass of the reactants.
The difference between the total mass of the products and the total mass of the reactants (mass defect) corresponds to the energy, which is called reaction energy. It can be calculated using Einstein's energy equation:
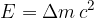
where:
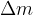 is the mass reaction defect which is given as:
is the mass reaction defect which is given as:
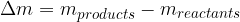
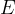 is the energy released or consumed in the reaction.
is the energy released or consumed in the reaction.
Since the mass defect is positive in exothermic reactions, the reaction energy 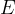 is also positive.
is also positive.
The concept of reaction energy is related to the concept of binding energy. Binding energy is used only for individual nuclei, while reaction energy is used for overall reactions that may involve several nuclei.
The most important types of exothermic reactions are:
splitting of heavier nuclei into lighter ones, this is called fission, and
combination of lighter nuclei into heavier one, this is called fusion.
Both types of reactions are characterized by the fact that the total mass of the products is always smaller than the total mass of the reactants.
Exothermic nuclear reactions are very important for humanity, as they are used to obtain large amounts of energy. Currently, we use nuclear reactors to obtain energy, in which controlled nuclear decay or nuclear fission takes place. A lot of attention is being paid to the development of new reactors in which controlled nuclear fusion or fusion would take place. With such reactors, even larger amounts of energy could be obtained.
Exothermic reactions release energy. The total mass of the products is therefore less than the total mass of the reactants. The difference in mass and the energy released during the reaction are connected by Einstein's energy equation.
Exothermic reactions include the decay of heavy nuclei and the fusion of light nuclei.
The exothermicity of the fusion of light nuclei and the splitting of heavier ones can be understood from the analysis of the diagram of specific binding energies for different nuclei:
Figure 1 shows the specific binding energy against the nucleus size. It helps us to understand two fundamental and already mentioned nuclear phenomena:
fission, that is, the breakdown of heavier nuclei into lighter ones, and
fusion, that is, the merging (combination) of lighter nuclei into heavier ones.
Let's get to know both processes in more detail.
Fission is a phenomenon where heavier nuclei break up into lighter ones. Why do breakups occur? The nucleons in the nucleus are held together by short-range nuclear forces. But when the nuclei become too large, the nuclear forces can no longer hold them together, so the nuclei break up into smaller nuclei.
In small nuclei, all the nucleons are close together. At short mutual distances, nuclear attractive forces dominate over electromagnetic repulsive forces (Figure 2).
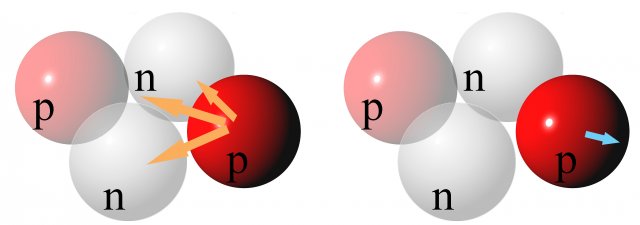
Figure 2: Schematic representation of the action of forces on one proton in a light nucleus (helium). Left - attractive nuclear forces between nucleons. Right - the repulsive electric force of the second proton
With each added nucleon, the attractive forces increase further. Nuclei are bound more and more strongly, which is expressed in an increase in the specific binding energy per nucleon (Figure 1). The most strongly bound nuclei have around 60 nucleons. This can also be confirmed from Figure 1, as the specific binding energy per nucleon has a maximum at 60 nucleons.
As the number of nucleons in the atomic nucleus continues to increase, so do the distances between the outer protons. At larger distances, however, the electric repulsive forces are considered to be greater than the attractive nuclear forces (Figure 3).
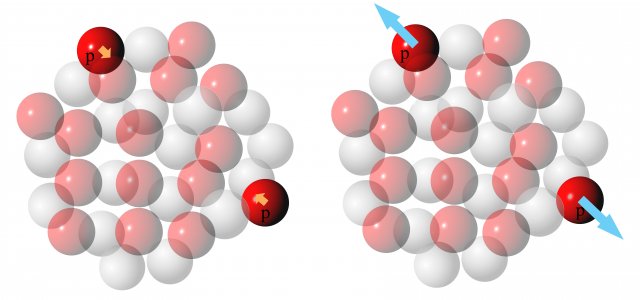
Figure 3: Schematic representation of the magnitude of the forces between two distant protons in a large nucleus. Left - attractive nuclear forces. Right - repulsive electric forces
The whole nucleus is therefore connected less and less firmly. This is reflected in the reduction of specific binding energy per nucleon in nuclei with more than 60 nucleons (Figure 1). While distant protons repel, they are still strongly bound to neighboring nucleons. It is therefore energetically advantageous for heavy nuclei to break up into several (usually two) smaller, but more strongly bound nuclei. This releases energy.
As an example, let's look at the energy balance of the hypothetical decay of the mercury nucleus. This nucleus has 200 nucleons. Suppose it decays into two nuclei of 100 nucleons each.
The greater the difference between the specific binding energy of the starting nucleus and the final products, the more energy is released in the decay.
Due to the released energy, the fission process is used to generate electricity in nuclear reactors. Fission is also the basis for the operation of physical nuclear bombs.
In the process of decay of medium-heavy nuclei into even lighter nuclei, this energy balance is no longer favourable. These nuclei have a higher specific binding energy, while the decay products have a lower one. Energy is consumed in these reactions.
Nuclear fusion is a process in which small nuclei combine into larger ones. As we have already described in the fission section, small nuclei are bound more strongly as nucleons are added. It is therefore energetically advantageous for small nuclei to combine. In doing so, they release energy.
Using the concrete example of the fusion of two small nuclei, let's see what the energy balance is in this reaction.
Fusion is the basic source of energy radiated by the Sun since the fusion of hydrogen into helium takes place in the core of the sun.
The principle of generating electricity in nuclear power plants is similar to that in thermal power plants which relies on heating water into steam. The steam then spins turbines connected to an electric generator that produces electricity. While coal combustion (a chemical reaction) is used in thermal power plants to heat water, nuclear power plants use the energy of nuclear reactions, specifically nuclear fission, to heat water.
Spontaneous radioactive decay of heavy nuclei takes place too slowly to heat reactors.
However, the decay of certain nuclei can also be triggered by bombarding them with neutrons. When a nucleus absorbs such a neutron, it enters an unstable state and decays. Such nuclei are called fissile.
Among the decay products, in addition to the newly formed nuclei, there are also neutrons, which can initiate new fissions upon further collisions. This process of self-renewing fissions is called a chain reaction.
Examples of such fissile nuclei are also uranium 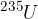 and polonium
and polonium 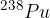 , which are most commonly used as nuclear fuel in nuclear power plants.
, which are most commonly used as nuclear fuel in nuclear power plants.
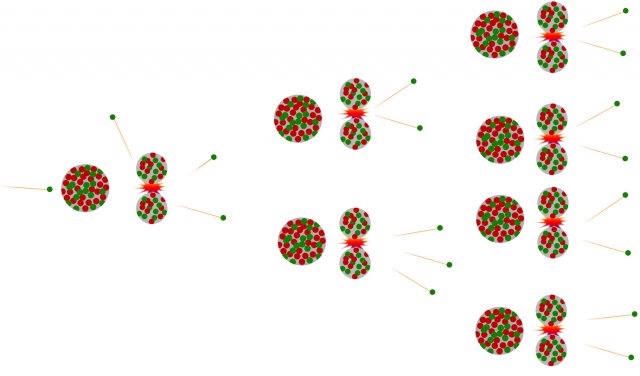
Figure 2: Chain reaction triggered by neutron capture and decay of the primary nucleus. With each decay, new neutrons are produced, which can trigger further fissions
Chain reactions can be:
Subcritical
Subcritical reactions are reactions that over time produce fewer and fewer neutrons that can initiate new reactions. The number of cleavages decreases with time and the reaction is extinguished.
Critical
In critical reactions, the number of fissions per time unit remains the same. The reaction is self-sustaining and a constant heat output is produced as a product.
Supercritical
When the number of fissions increases with time, the reaction is supercritical. More and more neutrons are produced, causing new fissions faster and faster. The heat output increases.
A critical reaction takes place in a nuclear reactor, which is maintained by the appropriate choice and design of the fuel, moderator, and control rods (substances that control the number and speed of neutrons and thus the speed of the reaction), as well as the coolant and the heat exchanger.
The supercritical reaction, however, expires after initiation in nuclear bombs. Within a very short time after the start of the reaction, the number of reactions and the energy released increase to such an extent that the bomb explodes.
For endothermic reactions, it is considered that:
energy is consumed, and
as a result, the mass of the products is greater than the mass of the reactants.
Since energy is consumed in a reaction, the particles entering the reaction must enter with some initial energy (usually kinetic). This enables the course of the reaction itself. This energy is usually contributed by the projectiles, which then react with the nuclei and start the reaction.
In endothermic reactions, energy is consumed. The total mass of the products is therefore greater than the total mass of the reactants. The energy required to start a reaction is usually contributed by the reactants in the form of initial kinetic energy.
Examples of endothermic reactions are:
fission of light nuclei, and
fusion of heavy nuclei.
In general, an endothermic reaction is any exothermic process that is forced to run in the opposite direction.