Personal collections


Some data for the work function energy 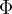 and the threshold frequency
and the threshold frequency 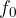 of some metal surfaces are given in the table below.
of some metal surfaces are given in the table below.
State what is meant by the threshold frequency.
Calculate the threshold frequency for platinum.
Electromagnetic radiation having a continuous spectrum of wavelengths between 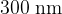 and
and 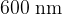 is incident, in turn, on each of the metals listed in the table above. Determine which metals, if any, will give rise to the emission of electrons.
is incident, in turn, on each of the metals listed in the table above. Determine which metals, if any, will give rise to the emission of electrons.
When light of a particular intensity and frequency is incident on a metal surface, electrons are emitted. State and explain the effect, if any, on the rate of emission of electrons from this surface for light of the same intensity and higher frequency.