Personal collections


By reference to the photoelectric effect, state what is meant by the threshold frequency.
Electrons are emitted from a metal surface when light of a particular wavelength is incident on the surface. Explain why the emitted electrons have a range of values of kinetic energy below a maximum value.
The wavelength of the incident radiation is 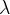 . The variation with
. The variation with 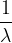 of the maximum kinetic energy
of the maximum kinetic energy 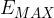 of electrons emitted from a metal surface is shown in the figure below.
of electrons emitted from a metal surface is shown in the figure below.
Use the figure above to determine, without reference to the work function energy, the threshold frequency 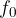 .
.
Use your answer in (i) to calculate the work function energy 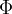 .
.
Caesium metal has a work function energy of 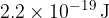 . On the axes of the figure above, sketch a graph to show the variation with
. On the axes of the figure above, sketch a graph to show the variation with 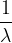 of
of 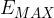 for caesium metal.
for caesium metal.