Personal collections


Polonium-211 (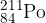 ) decays by alpha emission to form a stable isotope of lead (Pb).
) decays by alpha emission to form a stable isotope of lead (Pb).
Complete the equation for this decay.
The variation with time 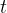 of the number of unstable nuclei
of the number of unstable nuclei  in a sample of polonium-211 is shown in the graph below.
in a sample of polonium-211 is shown in the graph below.
At time 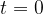 , the sample contains only polonium-211.
, the sample contains only polonium-211.
Use the graph above to determine the decay constant 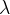 of polonium-211. Give a unit with your answer.
of polonium-211. Give a unit with your answer.
Use your answer in (b)(i) to calculate the activity at time 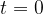 of the sample of polonium-211.
of the sample of polonium-211.
Each decay releases an alpha particle with energy 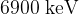 .
.
Calculate, in 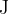 , the total amount of energy given to alpha particles that are emitted between time
, the total amount of energy given to alpha particles that are emitted between time 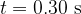 and time
and time 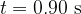 .
.
Suggest why the total amount of energy released by the decay process between time 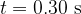 and time
and time 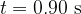 is greater than your answer in (c)(i).
is greater than your answer in (c)(i).