Personal collections


The specific latent heat of vaporisation is much greater than the specific latent heat of fusion for the same substance. Explain this, in terms of the spacing of molecules.
A heater supplies energy at a constant rate to 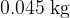 of a substance. The variation with time of the temperature of the substance is shown in the figure below. The substance is perfectly insulated from its surroundings.
of a substance. The variation with time of the temperature of the substance is shown in the figure below. The substance is perfectly insulated from its surroundings.
Determine the temperature at which the substance melts.
The power of the heater is 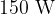 . Use data from the figure above to calculate, in
. Use data from the figure above to calculate, in 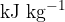 , the specific latent heat of vaporisation
, the specific latent heat of vaporisation 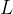 of the substance.
of the substance.
Suggest what can be deduced from the fact that section Q on the graph is less steep than section P.