Personal collections


The mass of nitrogen gas in a container is 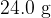 at a temperature of
at a temperature of 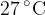 . The gas is cooled to its boiling point of
. The gas is cooled to its boiling point of 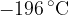 . Assume all the gas condenses to a liquid. For this change, the specific heat capacity of nitrogen gas is
. Assume all the gas condenses to a liquid. For this change, the specific heat capacity of nitrogen gas is 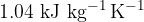 and the specific latent heat of vaporisation is
and the specific latent heat of vaporisation is 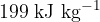 . Determine the thermal energy, in
. Determine the thermal energy, in 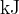 , removed from the nitrogen gas.
, removed from the nitrogen gas.