Personal collections


1 kg of water vapour at a temperature of 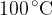 is placed in a thermally insulated container and 2 litres of ice-cold water at a temperature of
is placed in a thermally insulated container and 2 litres of ice-cold water at a temperature of 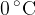 is poured into the container. What happens to the mixture when thermal equilibrium is established?
is poured into the container. What happens to the mixture when thermal equilibrium is established?
The specific heat capacity of water is 4200 J/kg K and the specific latent heat of vaporisation of water is 2.26 MJ/kg.