Personal collections


6 liters of water at a temperature of 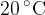 is placed in a thermally insulated container and 0.1 kg of steam at temperature
is placed in a thermally insulated container and 0.1 kg of steam at temperature 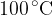 is introduced into the water. What happens to the mixture in the container?
is introduced into the water. What happens to the mixture in the container?
The specific heat capacity of water is 4200 J/kg K and the specific latent heat of vaporisation of water is 2.26 MJ/kg.