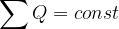In nature, we can observe phenomena that cannot be explained by knowledge of classical mechanics. These include lightning strikes, the glow of metal objects during a storm, attractive and repulsive forces between objects rubbed with a dry cloth, etc.
The ancient Greeks already noticed that a stick of amber, previously rubbed against fur, could attract small objects. They did not understand the phenomenon at the time, nor did they understand the nature of lightning. Amber means electron in ancient Greek. From this name, the name for a new field of physics later developed: electricity.
Today we know that all these phenomena have in common an electric charge (in electronics, we call it electricity). An electric charge creates an electric field through which forces are transmitted between the charges without their direct contact. In the chapter, Force as a vector, we called this phenomenon the action of force at a distance. The gravitational forces, made possible by the gravitational field, acts similarly at a distance.
Considering the fact that charged bodies attract or repel each other, we conclude that there are two types of charges: positive and negative charges. This is also where it differs from gravity because mass can only be positive, and the gravitational force is always directed only in such a way that masses attract each other.
An electric charge can be moving or at rest:
if it is moving, we call it an electric current;
if it is at rest, we are talking about electrostatics.
Basic knowledge of what an electric charge is and how it affects the environment will be learned in the following chapters.
What is an electric charge? An electric charge is the result of a lack or excess of electrons in a substance. In order to charge a body, we need to add or remove electrons from it.
An electron is a negatively charged elementary particle with the smallest possible electric charge. We call it a base charge.
Let's remember that the basic building blocks of all atoms are (for more, see the chapter, Atomic Structure):
electrons, electrically negatively charged particles;
protons, electrically positively charged particles;
neutrons, electrically neutral particles.
Atoms of a substance have the same number of negatively charged electrons and positively charged protons. Among them, there are electric forces, which are one of the most important forces (besides nuclear forces) inside the atom. The electric forces inside the atom are equal to the radial forces, which allow the electrons to stay around the positively charged nucleus.
Although electrical forces exist inside the atom, the charges of the electrons and protons cancel out on the outside. The atom is thus externally electrically neutral.
Even the substance itself, which consists of atoms and molecules, is externally neutral. It contains an equal number of positively and negatively charged particles. When, for example, a certain number of electrons were transferred to amber from a cloth by rubbing, the common neutrality of all amber atoms was destroyed. We say that we charged it with a negative electric charge.
If we observe matter from the outside (we do not go into the electric forces inside the atom), we see that the matter becomes electrically charged if electrons are added or taken away from it from the outside. The electric charge that a substance gets depends on the number of electrons that have been added or removed.
Today, we know that by rubbing amber with a dry cloth, basic particles of matter are transferred from the cloth to the amber, which are called electrons after the Greek name for amber. On the contrary, if we rub a glass rod with a dry cloth, we take away some of its electrons. The rod becomes positively charged, as there are now more protons than electrons in the substance.
The symbol for electric charge quantity is 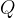 , and the unit is ampere second (
, and the unit is ampere second (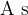 ) or Coulomb (
) or Coulomb ( ).
).
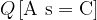
A proton (positively charged) and an electron (negatively charged) have the smallest possible charge quantity, which is called the base charge. It is denoted by  :
:
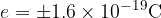
Since the electron is the smallest possible unit of charge, any electric charge quantity 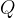 is always a multiple (N-times) of the base charge
is always a multiple (N-times) of the base charge  :
:
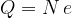
An electric charge quantity 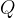 is always a multiple of the base charge
is always a multiple of the base charge  that an electron or a proton has in the nucleus:
that an electron or a proton has in the nucleus:
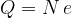
The charge of an electron is:
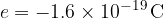
The charge of a proton is:
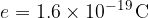
Substances are divided into:
conductors; they conduct electricity,
insulators; they do not conduct electricity,
semiconductors; they conduct electricity if certain external conditions are met.
In the next two subsections, we will deal with conductors and insulators.
Insulators are substances in which electrons cannot move, they can only rest. Nevertheless, they can be charged e.g. by rubbing with a silk cloth.
Insulators are:
Solids: The outer electrons in the atoms of an insulator are firmly bound to the nucleus and external forces can hardly "tear" them off the atoms. In insulators, electrons cannot move between atoms despite the presence of external forces. Insulators are resins (e.g. amber), glass, mica, paper, ceramics, etc.
Liquids: The same applies to liquids and even gases. Dry, clean air is an insulator, just as pure water is an insulator.
An insulator is a substance where the electrons are bound to the nucleus and cannot move.
Conductors are substances through which electrons can move. Unlike insulators, they cannot be charged by rubbing. We can charge them only by touching them with another charged body.
Conductors are:
Solids: In conductive solids, the outer electrons of the atoms can move freely between the atoms. Conductors are all metals: silver, copper, gold, iron, etc.
However, a substance that is basically an insulator can also become a conductor. This happens if the external forces on the electrons of the atoms are too great and "tear" them from the nucleus.
Fluids (liquids and gases): If we add a little acid or base to pure water or add salt, it becomes conductive. The molecules of the dissolved added substance become charged particles - ions, which can move under the influence of external forces. Ions can be negative (anions) or positive (cations). A liquid with ions of a substance is called an electrolyte.
See also the following chapters in inorganic chemistry:
Protolytic reactions - the behavior of acids and bases in water, and
Electrolytic dissociation - the behavior of salt in water.
Even clean air is basically an insulator. In the case of large forces on the electrons bound to the nucleus of atoms, the electrons can be "torn" out of the nucleus. Air molecules are ionized. "Freed" electrons and positively ionized atoms lacking electrons (cations) can now move freely. Such free movement is observed in phenomena such as lightning strikes or spark jumps.
The gas can also be ionized due to high temperatures, solar radiation, etc.
A conductor is a solid, liquid, or gaseous substance where carriers of electric charges (e.g. electrons in metals, ions in air, or liquids - electrolytes) can move freely.
A charge can e.g. transfer from the amber or glass rod to two light metal balls suspended side by side on a string. We notice that the balls:
attract if they are charged with opposite charges (e.g. the first with the help of an amber rod, the second with a glass rod);
repel if we have charged them with charges of the same kind (e.g. both with amber or both with a glass rod).
We conclude that like charges (positive or negative) repel, while unlike charges (positive and negative) attract.
Let's see what happens if we charge a metal sphere, while there is no other charged body nearby to affect the observed body. Since the electrons repel each other, the electrons will therefore be evenly distributed over the surface of the metal sphere. Also, the electrons will be pushed to the surface of the sphere, since they are the furthest away from the electrons on the other side of the sphere. The electrons are therefore on the surface, so we introduce a new quantity 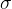 that measures the surface charge density:
that measures the surface charge density:
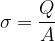
Its unit is:
Electric forces act between electric charges. Like charges repel, unlike charges attract.
If there is no other charged body nearby, the charge on an electrically conductive body is distributed evenly over the surface. The surface charge density 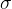 is given as:
is given as:
The fact that like charges repel each other is used in designing an instrument used to measure electric charge, this is called an electrometer (see Figure 3).
The electrometer works on the basis of repulsive forces between two metal sheets (leaves), on which like charges are evenly distributed. The charge is transferred to the leaves with the help of e.g. charged rods over the outer metal plate (top) as shown in Figure 3.
If there is no charge on the leaves, the angle between them is zero. At that time, only the torque of gravity acts on both leaves. When a charged body touches the outer plate, the charge is transferred and evenly distributed over both leaves. There is a repulsive electric force between them, so the leaves move apart. The angle they subtend is proportional to the electric charge.
Different versions of electrometers are possible.
Normally, we want as high an electrometer sensitivity as possible. The sensitivity tells us what is the minimum charge that can still be detected and cause a deflection.
To increase the sensitivity of the electrometer, the leaves should be as light as possible. This can be achieved by using thin gold foil (gold can theoretically be forged to a thickness of one molecule) - right image in Figure 3. An additional advantage of gold is that it does not oxidize and therefore the weight of the leaves does not change over time. In this way, we maintain the accuracy of the measurements in the long term.
The leaves can also be balanced with the help of the central axis - left image in Figure 3. In this way, we can arbitrarily reduce the sum of the torques of the force of gravity and increase the sensitivity of the electrometer. The torque of the force of gravity of both arms relative to the axis of rotation can be nullified.
An electric charge can be transferred from body to body. But the total charge is always conserved.
The law of conservation of electric charge states that when a charge is transferred from one body to another, the total charge is conserved. This is expressed mathematically as: